Abstract
Introduction Angioimmunoblastic T-cell lymphoma (AITL) is the second most common subtype of peripheral T-cell lymphoma (PTCL), accounting for 19% of all T-cell lymphoma cases. It usually presents with advanced disease, systemic symptoms, and immune deregulation. At present, cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) is the most common first-line chemotherapy regimen. Other chemotherapy regimens include CHOPE (etoposide plus CHOP), dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin), Hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and prednisone), and MINE (mesna, ifosfamide, mitoxantrone, etoposide). However, their therapeutic effects are not satisfactory, and most patients experience relapse or disease progression. The 5-year PFS of AITL is 13%-23%, and 5-year OS is 33%-36%. Chidamide, a benzamide histone deacetylase inhibitor (HDACi), is the first new drug to be approved for the treatment of relapsed or refractory PTCL in China. Lenalidomide is a second-generation immunomodulatory compound that targets tumor cells and the tumor microenvironment. About 70%-100% of the AITL patients have EBV infection, which may cause abnormal immune functions in hosts. EBV infects immunocytes such as B cells, T cells, and natural killer cells, and it impairs immune functions that can clear neoplastic cells from the body. Rituximab may eradicate EBV-B cells and activated B cells and may prevent the progression to AITL. When combined with rituximab, lenalidomide augmented antibody-dependent cell-mediated cytotoxicity by enhancing NK cells, which overcomes rituximab resistance in lymphoma patients. We performed this study to explore the efficacy and safety of rituximab, lenalidomide plus chidamide in relapsed/refractory AITL patients.
Methods Patients with relapsed/refractory AITL after at least first-line chemotherapy were treated with up to 6 cycles of a rituximab and lenalidomide plus chidamide (RLC) regimen every 3 weeks. Rituximab was intravenously administered on Day 1 at a dose of 375 mg/m2 in combination with lenalidomide (15 mg/day, Days 1-14) and chidamide (30 mg, twice a week). Imaging examinations were performed every two cycles to evaluate therapeutic efficiency. The primary endpoint of the study was progression-free survival (PFS). The secondary endpoints included overall response rate (ORR), overall survival (OS), and adverse events (AEs).
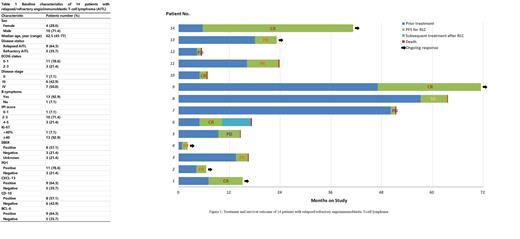
Results Between Aug 2019 and May 2022, a total of 14 patients with relapsed/refractory AITL were enrolled. On enrollment, 8 (57.1%) patients had received 1st-line chemotherapy, 5 (35.7%) patients had received 2nd-line chemotherapy, and 1 (7.1%) patient had received 3rd-line chemotherapy. The median age was 62.5 years (range 45-77) and 4 (28.6%) patients were female. Thirteen (92.9%) patients had stage III/IV disease and only 1 (7.1%) patient had stage II. Thirteen (92.9%) patients had B symptoms, including weight loss in 3 (21.4%) cases, night sweat in 1 (7.1%) case and fever in 9 (64.3%) cases. Eight (57.1%) patients had elevated serum lactate dehydrogenase (LDH), and 13 (92.9%) had IPI score≥2. All patients had completed RLC treatment for at least 1 cycle and underwent imaging assessment. The ORR was 71.4% (10/14) with a CR rate of 35.7% (5/14), while another 1 (7.1%) patient achieved stable disease (SD) and 3 (21.4%) patients achieved progressive disease (PD). Two patients received autologous hematopoietic stem cell transplantation as consolidation treatment after achieving CR from RLC chemotherapy. After a median follow-up of 24.4 months, 8 patients had died. The median PFS was 6.3 months (95% confidence interval <CI>, 4.6 to 8.0 months), and the median OS was 7.5 months (95% CI, 4.0 to 11.0 months).
No patient presented with Grade 5 AE. The most frequent all-grade hematological toxicity was leucopenia (35.7%, 5/14), followed by anemia (21.4%, 3/14) and thrombocytopenia (35.7%, 5/14). Other common toxicities included infusion reaction (14.3%, 2/14), skin rash (21.4%, 3/14), pulmonary pneumonia (42.9%, 6/14), and nausea (7.1%, 1/14). Dose reduction was performed in 4 patients.
Conclusions This study showed that the rituximab and lenalidomide plus chidamide (RLC) regimen is associated with high response rate and manageable toxicity in patients with relapsed/refractory AITL.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal